Are you passionate about shaping the future of patient care?
Ever since 1937, Ambu has been rethinking medical solutions to save lives and improve patient care. We believe independent research can contribute to this purpose by advancing medical and scientific knowledge.
With this in mind, we are inviting clinicians and researchers to apply for Investigator-Initiated Studies (IIS): independent research projects that explore Ambu products and procedures in real-world clinical settings.
Turn innovative ideas into evidence that matters
Join us in driving progress and improving outcomes for patients everywhere. Apply today.
If you are based in the US or Canada, please see our regional guideline.
Areas of Scientific Interest
Ambu supports Investigator-Initiated Studies that generate meaningful real-world evidence with a clinical, health economics, and sustainability focal point. Real-world evidence is critical for advancing clinical practice. It validates cost-effectiveness and patient outcomes, informs evidence-based decisions, and identifies usage trends and unmet needs—driving continuous device improvement and innovation.
Information Centre
Before applying
Areas of scientific interest
Ambu supports IISs that aim to improve the overall quality of patient care and generate meaningful real-world evidence, focusing on sustainability, health economics, and clinical outcomes.
The IIS must include one or more of Ambu’s products and be aligned with our areas of scientific interest.
Current areas of interest include:
- Clinical performance related to key Ambu product areas, including endoscopy, airway management, and patient monitoring
- Organizational impact, including workflow, cost and sustainability related to Ambu solutions
- Impact of digital technologies on clinical practice and organizational efficiency
Who can apply
We welcome clinicians, nurses, and researchers, including PhD and postdoctoral students, to apply for IIS support.
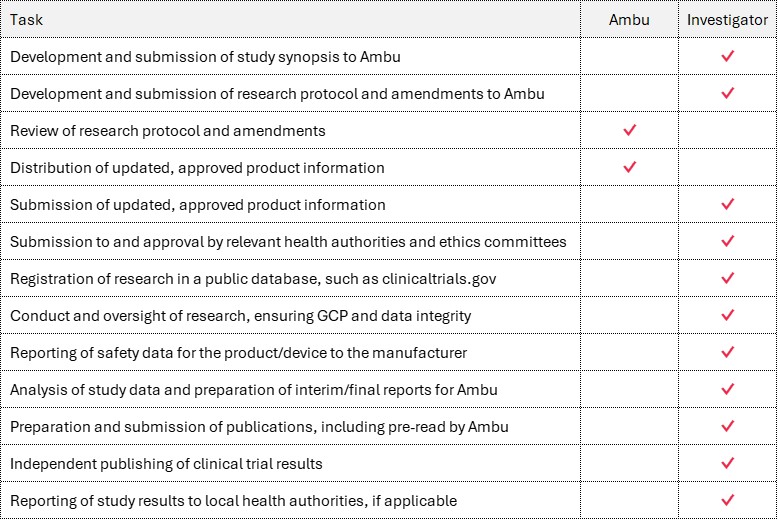
Key responsibilities of Ambu and the Investigator
In an Investigator-Initiated Study research is conceptualised, initiated, and conducted by an investigator who has full responsibility for the research program. The investigator and/or institution that employs the investigator maintains sponsor responsibility for the study. It is never Ambu.
Contractual responsibilities will be defined explicitly in an IIS Agreement prior to the start of the study.
Below is an overview of the most common tasks and responsibilities.
Types of support
All support is evaluated individually on a case-by-case basis to ensure compliance with ethical standards and transparency.
Support generally refers to contributions that support research, such as provision of study-related products, in-kind support; non-monetary contributions such as product training to ensure proper use, and/or financial support; directly related to the study or publication.
How to apply
Submitting your application
Fill out and submit the application form including all mandatory information. Our IIS team will contact you to request a copy of your study synopsis along with your most recent CV.
These data allow the Ambu IIS Review Board to conduct a thorough internal evaluation helping to assess scientific merit, investigator qualifications, and proper use of our products.
Deadlines
New IIS Applications are evaluated on a regular basis. There is no deadline for submitting your application.
Evaluation
Evaluation and approval
IIS applications are reviewed by the Ambu IIS Review Board. Approval is based on criteria such as scientific merit, relevance, alignment with research priorities, current scientific trends, and available resources.
Protocol
Should the IIS Review Board find our application interesting and relevant you will be required to share a draft-protocol for further evaluation. During this phase of the process, you most likely will be invited for a call.
Response time
Timelines for application evaluation and approval can vary. You can expect a response to your initial enquiry or application within 30 days.
Upon approval
IIS Agreement
Following approval, Ambu will draft an IIS Agreement that should be fully executed prior to any study activityor support taking place.
Registration and ethics approval
It is the investigator/institution's responsibility to report any clinical research to a clinical trials website such as ClinicalTrials.gov.
You must also submit an institutional Review Board IRB or ethics committee Letter of Approval/Letter of Exemption, if applicable.
During study
Progress updates
You may be asked to provide progress updates on a regular basis depending on the scope and length of the IIS. This requirement will be outlined in the IIS Agreement, including the frequency and format of the updates.
Adverse events
It is the investigator’s responsibility to report any study-related adverse events as required according to national and institutional reporting procedures.
Post-study and Dissemination
Return of unused products and/or funds
Depending on the scope of the study and the support granted, you may be required to return any unused investigational products and/or unspent funds. This requirement will be outlined in the IIS agreement where applicable.
Draft publication
The investigator is required to submit a draft of the publication to Ambu prior to applying for scientific congress/journal. The details and time frame will be outlined in the IIS agreement.
Publication and/or dissemination of results
Dissemination of the findings contributes to scientific advancement and transparency. The investigator is therefore encouraged to publish and/or present the results of the study in scientific journals or at relevant congresses.
For any questions specifically related to Investigator-Initiated Studies please contact us.
Application for Ambu Investigator-Initiated Study Support
To ensure your application is considered, please complete all mandatory fields with as much detail as possible. This helps the IIS Review Board evaluate your proposal thoroughly. Applications are reviewed on a rolling basis, and you can expect an initial response to your submission within 30 days.
If you are not ready to submit your application, we encourage you to visit our Information Centre for more details. If you have any questions or need assistance, please do not hesitate to contact us.


