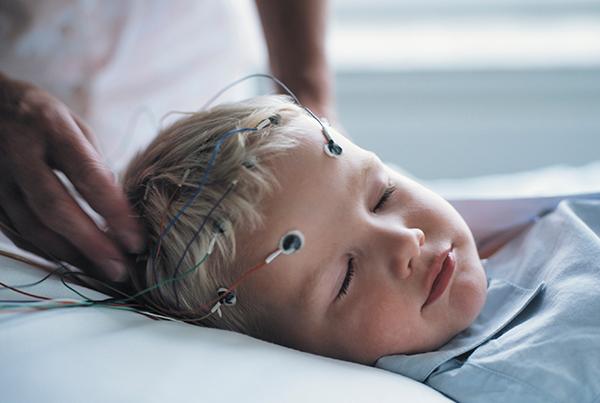
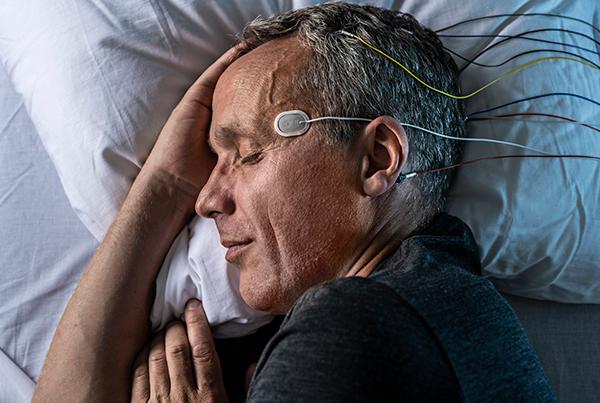
Ambu® Neuroline™ Cup
The Neuroline Cup is a fully single-patient use EEG Cup Electrode to minimize the risk of bacterial cross-contamination and to enhance departmental efficiency. The Neuroline Cup electrode is used for clinical EEG, EP and PSG examinations.
The Neuroline Single-Patient Cup electrode ensures stable signal quality and low impedance generated by the electrode.
Features & benefits
Ambu® Neuroline™ Cup
One Pack, One Patient
With a total of 15 options to choose from, you always get the number of electrodes needed for a procedure with less potential for wasted electrodes. Based on the array of electrodes used in standard procedures, including PSG and other EEG and EP applications, Neuroline Cup electrodes are available in 10, 15, 23, 25 and 27 pieces per pack.
The pack sizes give you a smoother preparation workflow, and the single-use range reduces the risk of cross-contamination.
Add a choice of 100, 150 and 200 cm variants and you have a comprehensive choice to suit most applications – using just one pack per patient.
- No need to open more pouches per patient
- Simply choose the number of electrodes required for specific procedures
- Easy identification with 12 different lead wire colours
- Single-use range minimises risk of cross-contamination
Suitable applications
Electroencephalogram (EEG)
Evoked potentials (EP)
Polysomnography (PSG)
Specifications
See datasheet for more specifications.
Sensor type
Silver/Silver chlorideLead wire length
100 cm (40"), 150 cm (60"), 200 cm (80")Connector type
M-connector (1.5 mm connector)Spare parts
There are no spare parts or accessories for this product.Downloads
Brochures
(1.31 MB - pdf)
Datasheets
(298.71 KB - pdf)
Instructions for use
(3.04 MB - pdf)
(1.66 MB - pdf)
Supplementary Information
(943.98 KB - pdf)
(2.38 MB - pdf)
(156.57 KB - pdf)
February 2023
Note: US: Rx only